How To Calculate Entropy Generation
Determine the heat production of entropy inside this motor for the time period τ 300 min of operation. The magnitude of the entropy generation irreversibility associated with each process can be determined by calculating the total entropy change for each case.
The entropy balance equation for a SISO process operating at steady-state that exchanges heat only with the surroundings is.
How to calculate entropy generation. The temperature at which heat transfer occurs is the surface temperature of the turbine 200 o C. S 0 NH 3 1925 Jmol K. Furthermore it includes the entropy of the system and the entropy of the surroundings.
Consider a system in contact with a heat reservoir during a reversible process. Using the Steam Property Calculator properties are determined using Inlet Pressure and the selected second parameter Temperature Specific Enthalpy Specific Entropy or Quality. If there is heat absorbed by the reservoir at temperature the change in entropy of the reservoir is In general reversible processes are accompanied by heat exchanges that occur at different temperatures.
Calculate the change in entropy associated with the Haber process for the production of ammonia from nitrogen and hydrogen gas. If the entropy generation is taken per unit time and per unit volume it is called the volumetric rate of entropy generation Φ. Recall the definition of Shannon entropy.
P x i Here X is the state eg. The total entropy change for a heat transfer process involving two reservoirs a source. Inlet Energy Flow Specific Enthalpy Mass Flow.
We can determine the entropy generation from an entropy balance on the turbine. 5 Irreversibility Entropy Changes and Lost Work. The x i values are the possible states eg.
1 2 3 4. The case involve heat transfer through a finite temperature difference and therefore it is irreversible. N 2 g 3 H 2 g 2 NH 3 g At 298K as a standard temperature.
Calculate the integral of dqT for the reversible path that you have devised. For your problem this procedure will. Number of symbols in the password S Size of the pool of unique possible symbols character set.
An electric motor has a volume of 250 10 3 m 3 and operates with a constant internal thermal entropy production rate per unit volume of σ Q 537 WKm3. The entropy change of a closed system is due to the entropy transfer accompanying heat transfer and the entropy generation within the system boundaries. Besides there are many equations to calculate entropy.
The Specific Enthalpy is then multiplied by the Mass Flow to get the Energy Flow. If the happening process is at a constant temperature then entropy will be Delta S_system fracq _revT Derivation of Entropy Formula Delta S is the change in entropy. Entropy is a function of state.
Determine the total entropy generation. Entropy Formula L Password Length. This will give you the change in entropy of the system for the irreversible path as well as for the reversible path.
This would include both the entropy generated inside the turbine and the entropy generated due to the irreversible nature of heat transfer through a finite temperature difference that is between T surf and T surr. Witte and Shamsundar 1983 proposed a thermodynamic efficiency concept for heat exchange devices. An entropy generation rate formula for a general fin and then applied the analytical methods and graphical results developed as a result for selecting optimum dimensions of fins.
If the high temperature reservoir is at and the lowtemperature reservoir is at the total entropy change is. The efficiency was written in terms of mean absolute. Figure 54Heat transfer between tworeservoirs.
The time derivative of the entropy is called the entropy generation rate and can be calculated from the laws of conservation of mass energy momentum and the second law of thermodynamics expressed as equality. The entropy change of the two reservoirs inFigure 54is the sum of the entropy change ofeach. If we used T HT T surr we would obtain the total entropy generation for the process.
H X i 1 n P x i log b. 1 1 1 1 1 1 1 2 10 10 10 10. Entropy change of the system Entropy transfer with heat Entropy generation.
The second law says that the entropy change must be equal to orgreater than zero. P x i is the probability that the random number X x i. This corresponds to the statement that heat mustflow from the higher.

What Is Entropy Generation Quora

Entropy Equation Summary Sheet 2 21 10
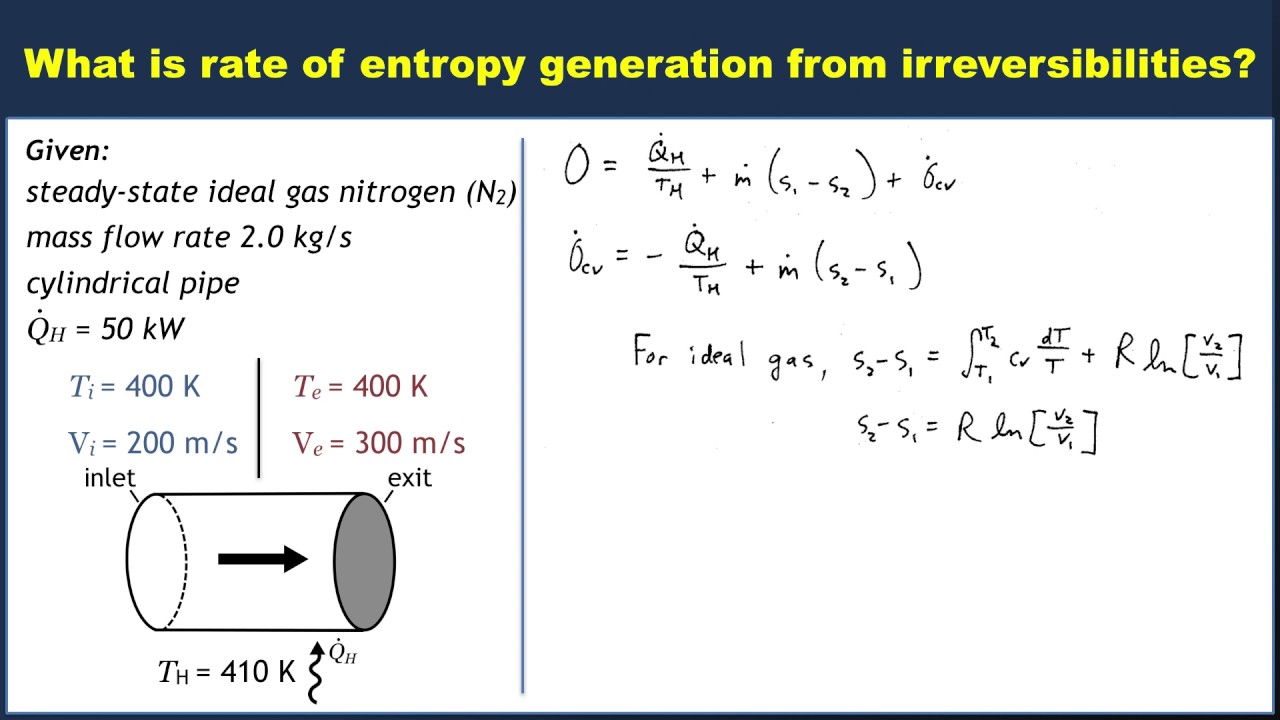
Example Entropy Balance In An Open System Youtube

Entropy Balance For Open Systems Ppt Video Online Download
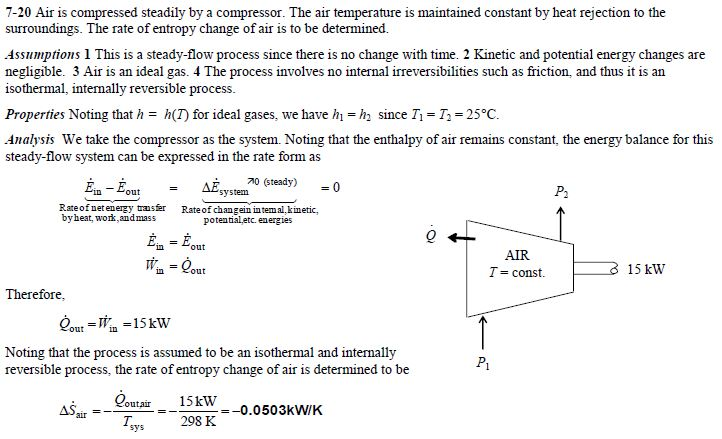
Solved For Problem 7 20 Also A Determine The Rate Of En Chegg Com
Calculating Entropy Generation And Bejan Number

Entropy Generation Rate An Overview Sciencedirect Topics

Entropy Equation Summary Sheet 2 21 10

Chem E 260 Entropy Balances On Open And

Does Positive Total Entropy Generation Means That The Entropy Generation Of The System And The Entropy Generation Of The Surroundings Are Positive Too Physics Stack Exchange

Entropy Generation Rate An Overview Sciencedirect Topics

Does Positive Total Entropy Generation Means That The Entropy Generation Of The System And The Entropy Generation Of The Surroundings Are Positive Too Physics Stack Exchange






Post a Comment for "How To Calculate Entropy Generation"